WILMINGTON, DE, Feb. 19, 2026 (GLOBE NEWSWIRE) — Disclaimer: This article is for informational purposes only. It is not medical advice. Body composition and wellness monitoring concerns should be evaluated by a qualified healthcare professional. This content does not diagnose, treat, cure, or prevent any disease. If you purchase through links in this article, a commission may be earned at no additional cost to you.
This release is an informational overview of publicly available disclosures for the Hume Body Pod and broader consumer research behavior within the at-home body composition analysis device category. The Hume Body Pod is a consumer wellness device. It is not intended to diagnose, treat, cure, or prevent any disease. Nothing in this content should be interpreted as medical advice, a product endorsement, or a performance claim.
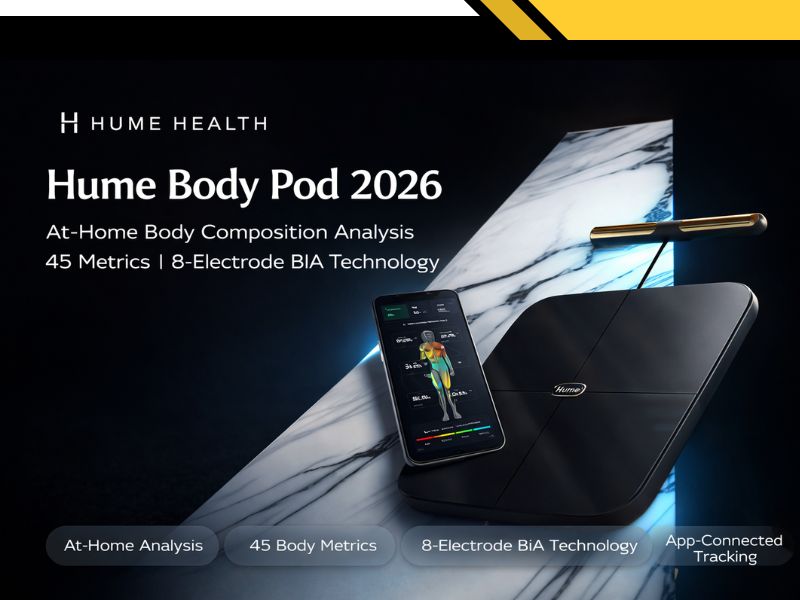
As consumer interest in at-home body composition analysis devices — and specifically in multi-frequency BIA technology, segmental body composition measurement, and multi-metric tracking beyond standard weight measurement — continues to grow heading into 2026, updated product disclosures for the Hume Body Pod have recently become publicly available. This article summarizes what Hume Health has disclosed about its product and provides category context for consumers who are actively researching their options.
Readers seeking primary-source detail can view the current Hume Body Pod offer (official Hume Health page) to review the company's complete product disclosures directly. This article does not assess product effectiveness or outcomes and focuses solely on publicly available disclosures.
Why Consumer Research in This Category Has Grown in 2026
Consumer interest in at-home body composition analysis devices that go beyond basic weight measurement has grown considerably heading into 2026. Across public forums, social platforms, and broader online discussion, consumer conversations about this category often center on electrode count, segmental measurement, multi-frequency BIA, validation disclosures, and how at-home body composition estimates differ from clinical reference methods — reflecting a consumer base that is asking far more detailed questions than simple weight tracking.
That shift reflects something real about what people want from body composition technology right now. Body fat percentage, visceral fat levels, skeletal muscle mass, metabolic age estimation, bone density indicators — none of these are niche interests anymore. They're mainstream research topics for people who want to understand not just what they weigh, but what their body's composition data might suggest about their overall wellness trajectory over time. Some companies have responded by publishing disclosures around multi-electrode configurations, segmental analysis capabilities, and proprietary metric methodologies — a framing that generates considerable consumer attention alongside appropriate scientific and regulatory scrutiny.
Understanding what a specific product actually discloses about its technology, methodology, and limitations — as distinct from what its marketing language implies — is a reasonable and necessary starting point for anyone doing serious research before deciding whether a product fits their needs. That is what this article is designed to provide.
What At-Home Body Composition Analysis Devices Typically Involve
At-home body composition analysis devices in the direct-to-consumer space are electronic devices — most commonly smart scales with integrated or handheld electrode components — that use embedded sensors to capture physiological signals when a user steps onto the platform or grips handheld sensors. That raw data is processed through proprietary algorithms that estimate various body composition metrics, which the user can review through a companion smartphone application over time. This overview does not assess or compare product performance across device formats or brands.
The most common measurement technology in this category is Bioelectrical Impedance Analysis (BIA), which works by sending low-level electrical signals through the body and analyzing how those signals travel through different tissue types. Devices in this space vary considerably in electrode count and configuration, the number and type of frequencies used to differentiate tissue types, whether they measure full-body or lower-body segments only, and the number of output metrics they report. The most transparent companies publish specific hardware details — electrode count, measurement frequency range, segmental analysis methodology — alongside regulatory certifications and accuracy validation disclosures. What's worth understanding is that those certifications describe regulatory and durability standards, not necessarily clinical measurement accuracy. That distinction matters when evaluating any product in this space.
The Scientific Context for BIA and Body Composition Measurement
Before examining what Hume Health discloses about its product specifications, it helps to establish the scientific context those specifications operate within — because the underlying research base for BIA technology is legitimate, even though individual consumer device implementations vary considerably in how faithfully they reflect it.
Bioelectrical Impedance Analysis (BIA) has been used in clinical and research settings for several decades and has a substantial research history in the body composition assessment literature. Research discussions in this space often distinguish between single-frequency and multi-frequency approaches, between whole-body estimates and segmental configurations that assess individual body regions independently, and between foot-only electrode setups and full-body configurations that include handheld sensors. Some studies suggest that multi-frequency, multi-electrode approaches may produce different results than single-frequency or foot-only configurations — though results vary across study designs, populations, and device implementations.
The broader concept of tracking body composition metrics over time for wellness monitoring purposes is well-established in the nutrition and exercise science literature. Dual-energy X-ray absorptiometry (DEXA) is widely regarded as a clinical reference standard for body composition measurement. Consumer-grade BIA devices operate on different technology and are generally understood to produce estimates rather than clinical-grade measurements — a distinction that matters when setting expectations for any product in this category.
One important caveat is worth stating plainly: the underlying science behind BIA technology is well-documented in peer-reviewed research. That does not validate any specific company's proprietary algorithm, and it does not guarantee that any given consumer device produces clinically meaningful outputs. Both things can be true at once — the underlying technology has a real research foundation, and consumer device implementations still require independent evaluation. This article does not evaluate scientific superiority claims or validate any specific company algorithm; it summarizes what Hume Health publishes about the Hume Body Pod and encourages readers to review the company's referenced materials and any cited validation documentation directly.
Readers seeking broader context on how Hume Health has approached multi-sensor health monitoring across its product line may find this previously published overview of Hume Health's wearable health monitoring disclosures useful as background.
What Hume Health Discloses About the Hume Body Pod Hardware
According to publicly available disclosures on the Hume Health website, the Hume Body Pod is a smart scale and body composition analyzer that includes both a platform base and handheld sensors. The company describes the device as using an 8-electrode system that sends bioelectrical signals through the body at multiple frequencies. Hume Health refers to this as a multi-frequency BIA approach designed to measure different tissue types across five body segments: left arm, right arm, torso, left leg, and right leg.
The company's published technical specifications describe the following: a weight range of 5 to 200 kg (up to 400 lbs), a weight precision of 50g, a 2.8-inch display, Bluetooth connectivity, and a rechargeable Li-ion battery. Platform dimensions are listed as 12.7 x 12.7 x 1.1 inches. Hume Health describes the device as incorporating what it refers to as a “medical grade measurement chip.” This reflects the company's product description and does not constitute an independent verification of regulatory classification or medical device status. The device is described by the company as supporting up to 24 individual user accounts.
Hume Health also discloses that the device has received FCC certification. Hume Health references an independent accuracy study conducted by Socotech and provides access to an on-page report for readers seeking methodological detail. This article does not independently validate study design or performance claims.
What Data Metrics the Hume Body Pod Is Disclosed to Track
Per company disclosures, the Hume Body Pod is described as tracking 45 body composition metrics. The company lists examples of tracked metrics including: body fat percentage, skeletal muscle mass, visceral fat, bone density, metabolic age, body water composition, and segmental analysis for individual limbs and torso. Hume Health also discloses that the device includes a heart rate sensor.
This overview summarizes what the company has publicly disclosed and does not verify, evaluate, or independently confirm any of these disclosures. These outputs are derived from the company's proprietary algorithms applied to the raw BIA sensor data — they are not clinical measurements — and are not intended to diagnose, treat, cure, or prevent any disease.
Application Access, Subscription Structure, and Pricing
Whether ongoing subscription access is required to see your own tracked data is one of the more practical considerations in this category — and Hume Health is reasonably direct about how their model works.
Hume Health discloses that the Hume Body Pod pairs with a free companion app (Hume Health app) available on iOS and Android. Per company disclosures, the free tier provides access to core body composition metrics with no subscription required. The company states the app supports integration with Apple Health, Google Health Connect, Fitbit, and Garmin platforms.
An optional premium in-app subscription is available at $9.99 per month, which the company states includes features such as personalized coaching, weekly health reports, and nutrition tracking. The company states premium features are not required to use the scale or access basic body composition data in the app.
Hume Health also discloses that the device is described as HSA/FSA eligible for U.S. customers — a detail worth noting for consumers who may be able to apply pre-tax healthcare funds toward the purchase, though individual eligibility should be confirmed with your own plan administrator. Pricing is subject to change; verify current pricing directly on the official Hume Body Pod product page before deciding.
What Is Currently Available and What Is Described as Planned or Premium
Based on publicly available disclosures from Hume Health as of February 19, 2026, the following reflects what the company states is currently available and what is described as premium or planned functionality.
Currently available per Hume Health disclosures: 8-electrode segmental BIA body composition analysis, 45 tracked body composition metrics, heart rate measurement, weight tracking with 50g precision, 2.8-inch display, Bluetooth connectivity, rechargeable battery, support for up to 24 user accounts, free companion app with core metric access, integration with Apple Health, Google Health Connect, Fitbit, and Garmin, optional premium subscription at $9.99 per month, and HSA/FSA eligibility status.
Described as premium subscription features per Hume Health disclosures: personalized coaching, weekly health reports, and nutrition tracking. Per the company's disclosures, these features are available at $9.99 per month and are not required to use the device or access basic body composition data.
Consumers should verify current feature availability and pricing directly on the official product page before deciding whether this product fits their needs.
Data Privacy and Ownership Disclosures
Data privacy deserves more than a passing mention when you're talking about a device that tracks sensitive health metrics like body fat percentage, visceral fat, and metabolic age. If data privacy matters to you — and for most people researching devices in this category, it does — take the time to review Hume Health's full terms of service directly. Those documents provide the most complete picture of how your data is collected, processed, stored, and used in connection with the service.
Important Limitations Consumers Should Understand
The following are factual characteristics of this product category — not criticisms of any single company. They apply broadly to direct-to-consumer BIA body composition analysis devices, and any company operating with real transparency should be willing to acknowledge them. If you're doing serious research, weighing each of these will help you set realistic expectations before deciding whether any product in this space is the right fit for you.
BIA accuracy is affected by hydration status and timing. Bioelectrical impedance analysis measures how electrical signals travel through body tissue — and water is the primary conductor. Your hydration level at the time of measurement can meaningfully affect your readings. Most body composition experts recommend measuring at the same time each day under consistent conditions — first thing in the morning, before eating or drinking — to minimize variability. A single reading taken after a large meal or an intense workout may not reflect your baseline body composition accurately.
There is no universal clinical standard for consumer BIA device accuracy. Different companies use different electrode configurations, different frequencies, and different proprietary algorithms to produce their body composition estimates. Metrics from one device are not directly comparable to metrics from another, and none are equivalent to clinical body composition assessments such as DEXA scans performed in medical or research settings.
Electrode contact consistency affects measurement quality. Multi-electrode devices that include both platform and handheld sensors require consistent contact between the skin and all electrode points. Dry skin, calluses, improper foot or hand placement, and movement during measurement can all affect signal quality and output consistency. Companies in this space generally recommend consistent positioning and clean, dry skin for optimal results.
Trend data is more meaningful than any single reading. The metrics that matter most in this category come from patterns observed over weeks and months, not from individual snapshots. One off day doesn't tell you much; what the data shows over the course of several weeks is where the real signal lives. Companies in this space, including Hume Health, generally frame their metrics as trend-oriented for exactly this reason.
Proprietary metrics cannot be independently verified. The 45 body composition metrics the Hume Body Pod reports are generated by Hume Health's internal algorithms. The full methodology behind those calculations — including how raw impedance data gets converted into specific metric outputs — is not fully disclosed in standard product descriptions. When you interpret those outputs, you're placing trust in the company's modeling and referenced validation data. That's not unusual in this category, but it's worth understanding going in.
The term “medical grade” in product descriptions is not a regulatory classification. Hume Health describes the device as incorporating a “medical grade measurement chip.” This is the company's product description language. It does not constitute FDA classification as a medical device, and it should not be interpreted as an indication of clinical-grade accuracy or regulatory approval for medical diagnostic use. The Hume Body Pod is a consumer wellness device.
Consumer body composition devices are not substitutes for medical evaluation. If you're experiencing physical symptoms that concern you, a body composition reading is not the right next step — a conversation with a qualified healthcare professional is. No consumer device replaces clinical assessment, and no tracked metric should be the reason you delay seeking care for something that warrants it.
Refund Policy and Customer Support Disclosures
According to published company policies, Hume Health describes a 45-day refund policy and provides customer service contact methods on its website. One detail consumers may want to note is that the company's published return policy states that devices must be in like-new condition and in original packaging, and must have been purchased directly from Hume.
The company discloses that return shipping costs are covered by the customer unless the product is confirmed defective following troubleshooting. Refund processing timing is described by the company as 5 to 7 business days after the return is received and approved.
Hume Health also discloses a standard 1-year warranty included with purchase, with an optional 10-year warranty upgrade available at additional cost. Readers interested in complete warranty terms are encouraged to review the company's published warranty disclosures directly on the official product page.
Frequently Asked Questions Based on Publicly Available Hume Health Disclosures
Is the Hume Body Pod a medical device? No. Per Hume Health's own disclosures, the Hume Body Pod is a consumer wellness device that is not intended to diagnose, treat, cure, or prevent any disease. The company's use of the term “medical grade measurement chip” reflects product description language, not FDA regulatory classification. Its outputs are for informational purposes and are not a substitute for professional medical advice.
What does the Hume Body Pod actually measure? Per company disclosures, the device uses an 8-electrode multi-frequency BIA system to analyze body composition across five body segments. The company states it tracks 45 metrics including body fat percentage, skeletal muscle mass, visceral fat, bone density, metabolic age, and body water composition. It also includes a heart rate sensor. These are proprietary algorithm-derived outputs, not clinical measurements.
How does it compare to a DEXA scan? The Hume Body Pod uses BIA technology, which is a different methodology than dual-energy X-ray absorptiometry (DEXA). DEXA is widely regarded as a clinical reference standard. Consumer-grade BIA devices operate on different technology and are generally understood to produce estimates rather than clinical-grade measurements. Research discussions in this space often distinguish between multi-frequency, multi-electrode configurations — like the one Hume Health describes — and simpler configurations, though results vary across studies and populations. Hume Health references an independent accuracy study by Socotech; consumers seeking methodological detail should review that report directly.
Do I need a subscription to use it? Per Hume Health's disclosures, no subscription is required to use the device or access core body composition metrics through the free application tier. An optional premium subscription is available at $9.99 per month for those who want additional features including personalized coaching and nutrition tracking.
How many users can share one device? Per company disclosures, the Hume Body Pod supports up to 24 individual user accounts through the companion app.
What is the return process?Hume Health describes a 45-day refund window. Devices must be in like-new condition and in original packaging, and must have been purchased directly from Hume. Return shipping costs are the customer's responsibility unless the product is confirmed defective. Refunds are typically processed within 5-7 business days of approval. Full terms and conditions are available on the company's published returns policy page.
Is it compatible with other health apps? Per company disclosures, the Hume Health companion app supports integration with Apple Health, Google Health Connect, Fitbit, and Garmin platforms.
Who May Want to Consult a Healthcare Professional
While consumer body composition analysis devices are widely available, certain individuals should talk with a qualified healthcare professional before using a BIA-based device. That includes people with implanted cardiac devices such as pacemakers or ICDs, anyone managing chronic health conditions, those undergoing active medical treatment, people who are pregnant or nursing, and individuals managing conditions that significantly affect fluid balance or body composition. Hume Health's published FAQ notes that its BIA technology has been studied in the context of cardiac implantable electronic devices, but if you fall into any of these groups, personalized guidance from your healthcare provider is the right first step.
More broadly, if you're experiencing symptoms that concern you, the right next step is a qualified provider — not a wellness device reading. Body composition data can be a useful conversation starter with your physician, but it is not a replacement for clinical judgment.
Readers who want to review Hume Health's full product disclosures at the primary source can do so here: view the current Hume Body Pod offer (official Hume Health page).
Consumers interested in Hume Health's broader health monitoring ecosystem — including its wearable health monitoring device platform featuring biological age estimation, Metabolic Momentum tracking, and longevity-oriented data analysis — can review a previously published disclosure overview covering Hume Health's wearable monitoring disclosures, which provides additional context on the company's product development and market positioning.
Contact: support@myhumehealth.com
Live Chat Mon-Sunday 6 am-6 pm
Disclaimer: This article is for informational purposes only and does not constitute medical advice. The Hume Body Pod is a consumer wellness device and is not intended to diagnose, treat, cure, or prevent any disease. The insights and data provided by the Hume Body Pod are not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. If you purchase through links in this article, a commission may be earned at no additional cost to you.
Affiliate Disclosure: If you purchase products through links in this article, a commission may be earned at no additional cost to you. Always confirm the latest details directly with the official brand before making any decisions.
Medical Disclaimer: This content is for informational purposes only and is not a substitute for professional medical advice. The Hume Body Pod is described by the company as a consumer wellness device and is not intended to diagnose, treat, cure, or prevent any disease. Always consult a qualified healthcare professional regarding medical concerns.
Product & Pricing Disclaimer: Product availability, feature availability, customer experiences, and pricing may vary. Always confirm current specifications, policies, and pricing directly with the official source before making any decisions.
Publisher Responsibility Disclaimer: The publisher of this article has made every effort to ensure accuracy at the time of publication. We do not accept responsibility for errors, omissions, or outcomes resulting from the use of the information provided. Readers are encouraged to verify all details directly with the official source.
References: Bioelectrical Impedance Analysis (BIA) has been discussed in peer-reviewed body composition literature including the European Journal of Clinical Nutrition, Journal of Clinical Densitometry, and Nutrients, among other publications. Socotech independent accuracy study referenced by Hume Health on product page. Readers are encouraged to review primary research sources directly for methodological detail.

Contact: support@myhumehealth.comLive Chat Mon-Sunday 6 am-6 pm
